You are here
Testing of Ebola vaccine is underway in Sierra Leone
Tue, 2015-04-14 16:58 — mike kraftUSA TODAY by Liz Szabo April 14, 2015
Sierra Leone has begun testing an experimental Ebola vaccine, officials of the Centers for Disease Control and Prevention announced Tuesday.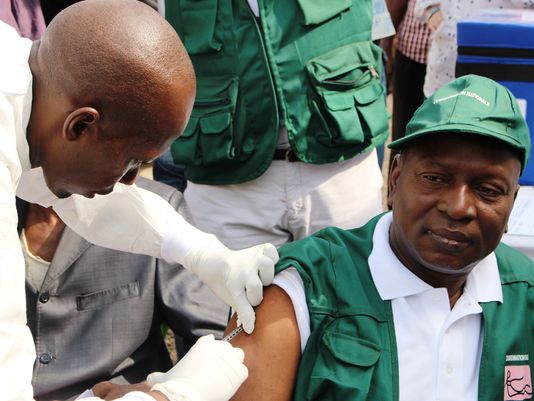 (Photo: CELLOU BINANI, AFP/Getty Images)
(Photo: CELLOU BINANI, AFP/Getty Images)
The $25 million study, funded through $5.4 billion in Ebola aid authorized by Congress, will test vaccines on 6,000 "front-line workers," including doctors, nurses, burial workers and others, who are at highest risk of the disease.
But with only a handful of new Ebola cases being reported now in Sierra Leone, it may be difficult to get a clear answer on whether the vaccine actually works, the CDC acknowledges. If there are no new cases of Ebola among vaccinated volunteers, for example, researchers won't know if that's the result of the immunizations or because the outbreak has faded....
The vaccine being rolled out in Sierra Leone was developed by the Public Health Agency of Canada and licensed to NewLink Genetics, which has licensed the vaccine to Merck. The vaccine has been tested on 800 people in Africa, Canada, Europe and the USA.
A second Ebola vaccine, developed at the National Institutes of Health and GlaxoSmithKline, is also in clinical trials in West Africa.
Read complete story.
http://www.usatoday.com/story/news/2015/04/14/ebola-vaccine-sierra-leone/25763899/

Recent Comments