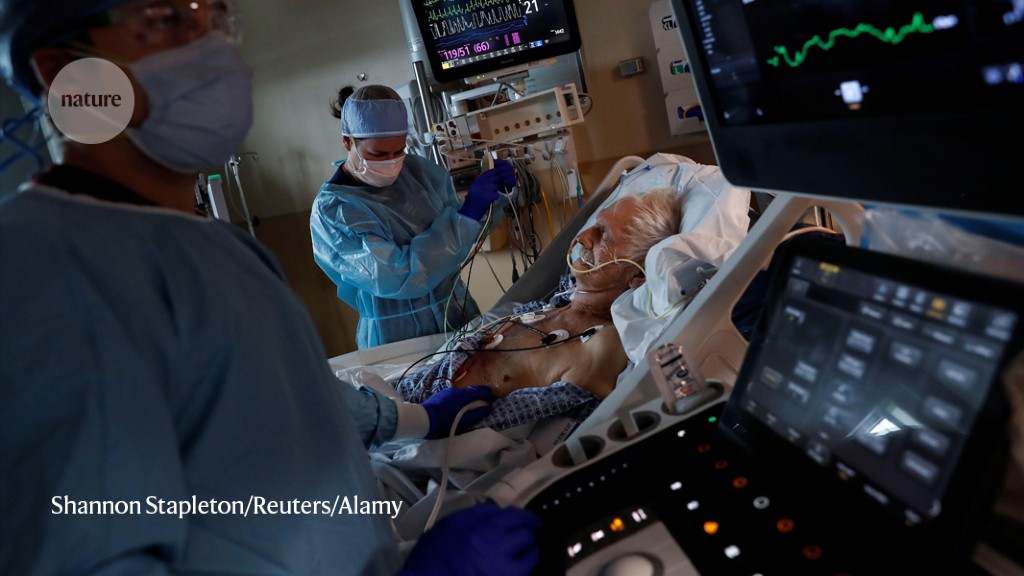
A landmark programme to test potential COVID-19 therapies in dozens of countries is restarting with a fresh roster of treatments — this time aimed at tempering the raging immune responses that can worsen severe disease.
The clinical trial, named Solidarity and coordinated by the World Health Organization (WHO), will test three drugs that dampen inflammation, an approach that has already shown promise in people hospitalized with COVID-19.
All three drugs were carefully chosen on the basis of the promise they showed in smaller clinical trials and widespread availability, says John-Arne Røttingen, scientific director of the Norwegian Institute of Public Health and chair of the Solidarity trial's international steering committee. “You need at least promising signals that some of them will work,” he says. “And we need study drugs that we can deliver in a broad group of countries.”
When the WHO launched Solidarity in March 2020, the study was focused on antiviral drugs. By October, the trial had enrolled more than 11,000 participants hospitalized with COVID-19 in 30 countries. But it also found that none of the four drugs that it tested (remdesivir, interferon, the malaria drug hydroxychloroquine, and a combination of HIV drugs called lopinavir and ritonavir) saved lives or shortened hospital stays1.
“None of the antivirals have shown strong effects in hospitalized patients,” says Røttingen. “The emerging consensus is that it’s too late. Where the antiviral medications could have a benefit is quickly after a positive test.”
Now, after a pause to sort out which therapies to try next, the trial hopes to focus instead on reining in immune responses that can contribute to severe forms of COVID-19. ...



Recent Comments